Molecular Imaging
Providing detailed images of the physiological, cellular and molecular state of the body.
Molecular imaging agents (radiopharmaceuticals) are smart compounds developed specifically for a particular tissue, organ, or molecular process. Such diagnostic radiopharmaceuticals are labeled with positron-emitting radionuclides (e.g., 18F) and distribute throughout the body. After intravenous injection, they disperse throughout the body and accumulate in disease areas. Positron emission tomography (PET), a noninvasive imaging technique, can be used to obtain images of the distribution of labeled tracers.
Molecular imaging offers unique insights into the human body that enable physicians to improve diagnosis and personalize patient care. It can provide information that is not available with other imaging modalities or that would require more invasive procedures such as biopsy or surgery. Molecular imaging can detect disease in its earliest stages and pinpoint its exact location, often before symptoms appear or abnormalities can be detected with other diagnostic tests.


Neuroimaging
Providing an opportunity to directly visualize and measure brain function and to depict pathophysiological processes of neurodegenerative diseases.
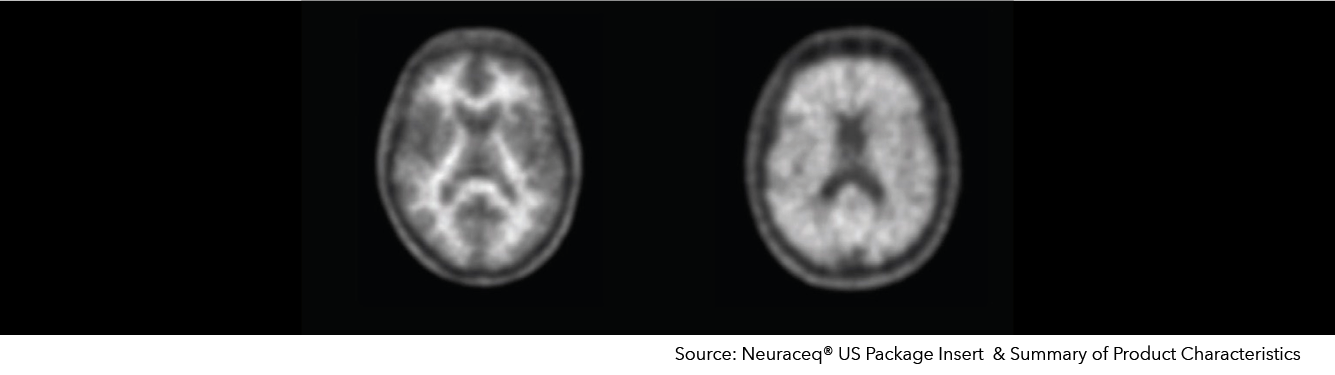
Neuraceq® – Beta Amyloid Imaging
Extracellular deposits of β-amyloid peptides (Aß) (or plaques) are one of the pathological hallmarks of Alzheimer's disease (AD). The recent development of molecular imaging tracers that bind to Aß plaques in the brain has enabled in vivo detection of Aß plaque deposits by PET. The noninvasive detection of Aß deposits may potentially contribute to better diagnosis and management of patients with cognitive impairment suspected of having neurodegenerative diseases. In addition, confirming the presence of Aß deposition in subjects and monitoring changes in Aß deposition could be critical during therapeutic trials specifically aimed at removing Aß deposits in the brain.
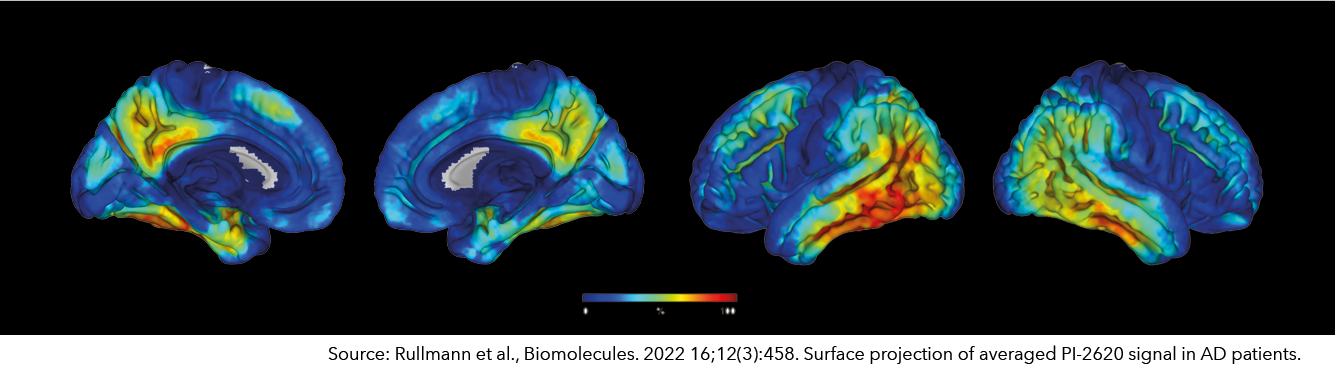
18F-PI-2620 – Tau Imaging
Abnormal accumulation of misfolded tau protein underlies several neurodegenerative diseases and is associated with various clinical syndromes. In addition to Alzheimer's disease (AD), several neurodegenerative disorders have been described in which the deposition of tau aggregates is a dominant pathology. These include atypical Parkinson's syndromes such as progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS), chronic traumatic encephalopathy, and some variants of frontotemporal lobar degeneration or subtypes of frontotemporal dementia.
Pl-2620 is a small molecule designed for binding and PET imaging of aggregated tau protein in the human brain. This innovative and differentiated diagnostic tool is available to physicians and patients worldwide and can already be used as an investigational tracer in research studies with tauopathy patients.
FDA Fast Track Designation
Life Molecular Imaging has received FDA Fast Track for clinical development of PI-2620 tau PET imaging in patients being evaluated for Alzheimer's disease (AD), progressive supranuclear palsy (PSP), or corticobasal degeneration (CBD). While we continue to advance our research and development efforts, please note that Expanded Access is not currently available for PI-2620.
For selected publications please go to.
*Under clinical investigation – not yet approved by regulatory agencies
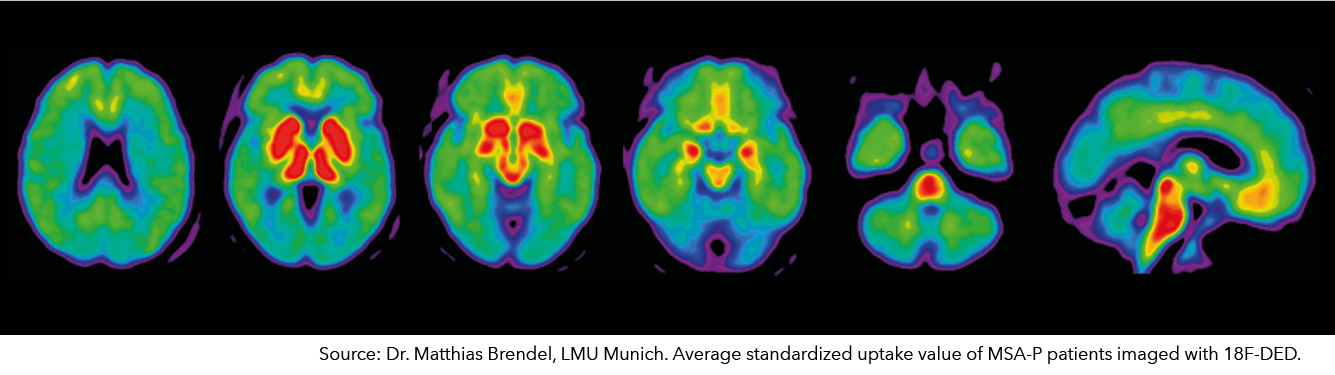
18F-DED – MAO-B Imaging
As an early component of neuroinflammation, astrogliosis is implicated in neurodegenerative diseases such as Alzheimer's disease (AD), multiple sclerosis, amyotrophic lateral sclerosis, and Parkinson's disease. PET imaging of activated astrocytes during neuroinflammation could improve characterization and monitoring of disease progression and therapy.
18F-labeled deuterated deprenyl (18F-DED) is a neuroimaging product candidate that specifically targets activated astrocytes during neuroinflammation..
For selected publications please go to.
*Under clinical investigation – not yet approved by regulatory agencies
Cardiovascular Imaging
Molecular imaging also has potential diagnostic applications in cardiovascular diseases. Critical components of a pathology can be selectively visualized and exploited using targeted molecular imaging approaches.
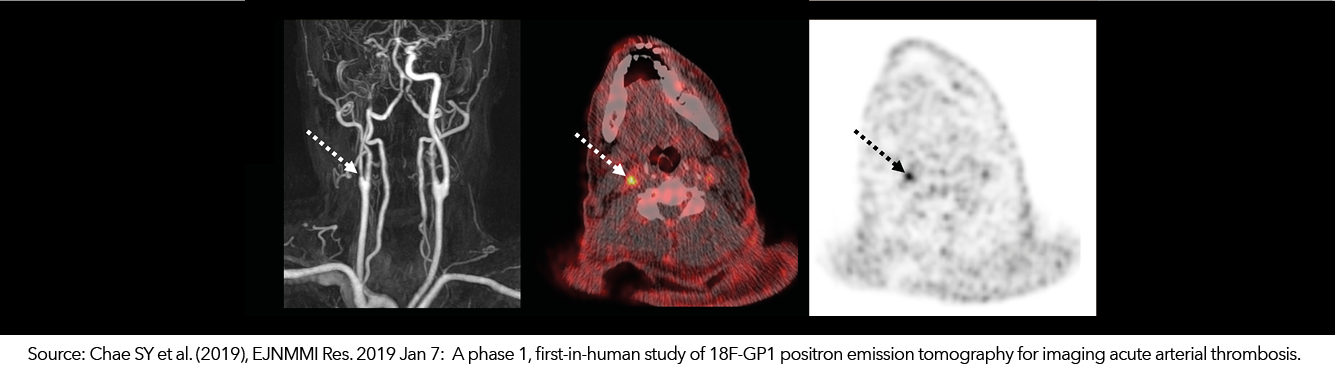
18F-GP1 – Thrombus Imaging
Life Molecular Imaging and its collaborators are studying 18F-GP1, a small molecule labeled with fluorine-18. It binds with high affinity to activated GPllb/llla receptors, which represent one of the key processes in thrombus formation and progression. 18F-GP1 is the first PET tracer shown to be able to detect acute thromboembolic events in patients.
For selected publications please go to.
*Under clinical investigation – not yet approved by regulatory agencies
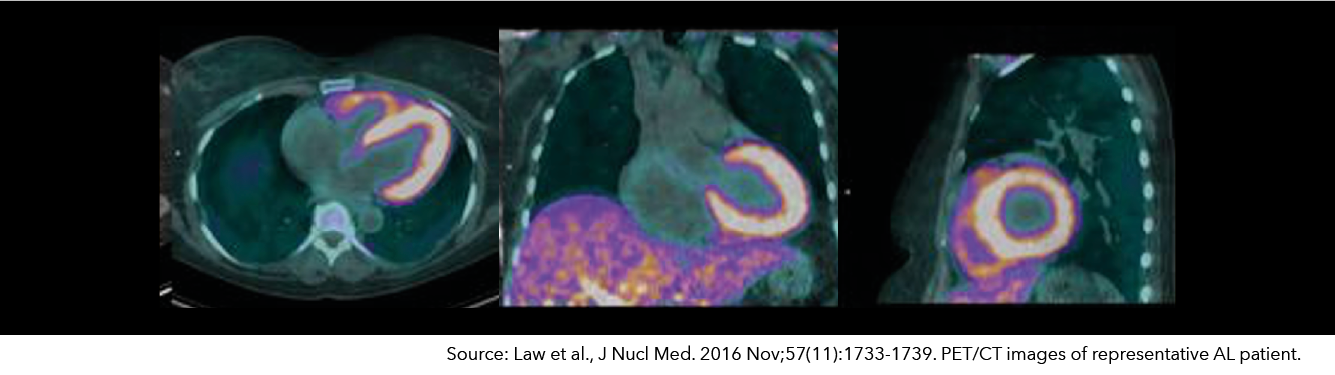
Florbetaben – Cardiac Amyloid Imaging
Cardiac amyloidosis is a life-threatening, progressive, infiltrating, rare disease that is often overlooked as a cause of heart failure. Multiple proteins with unstable structures misfold and aggregate to form amyloid fibrils that are deposited in the heart and other organs. Florbetaben is an 18F-labeled stilbene derivative that binds to amyloid deposits and is studied in patients with suspected cardiac amyloidosis.
Life Molecular Imaging has received FDA Fast Track for clinical development of [18F]Florbetaben PET imaging for the diagnosis of AL and ATTR cardiac amyloidosis. Expanded Access for [18F]Florbetaben in this indication is available under certain conditions. Neuraceq® is registered for PET imaging of B-amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) and other causes of cognitive impairment.
View the press release announcing the FDA Fast Track designation here.
Complete the Expanded Access & Compassionate Use Request Form here.
Contact us at expaccess[at]life-mi.com for more information.
For selected publications please go to.
*Under clinical investigation – not yet approved by regulatory agencies
Pharma Collaborations
A partner for pharma sponsors in pre-clinical and clinical studies
Life Molecular Imaging (LMI) partners with pharmaceutical companies to provide PET imaging radiotracers and services for therapeutic clinical trials – particularly in the neurodegenerative space – to help evaluate the effectiveness of disease modifying drugs for the treatment of Alzheimer’s disease (AD) and other diseases (e.g. non-AD tauopathies). For more than 10 years, LMI has provided global PET tracer supply management (e.g. Neuraceq®) and services in multiple countries around the globe with our extensive supply network for various Phase 1- 3 clinical trials.

As a partner, LMI provides:
• Single entity contracting to sponsors by providing access to LMI license partner product instead of Sponsor contracting with several different license holders
• High quality products, services and information in all processes, simplifying the component of
conducting complex trials for the sponsors
• Amyloid and tau products that meet cGMP quality standards
• Training and education to hundreds of imaging sites in ordering, receiving, and handling of
radioactive PET imaging tracers for specific pharma AD trials
• Regulatory support to sponsors in their clinical trial applications for submissions in
participating countries
• A dedicated team throughout the trial timeline – you have our support

LMI is unique as a partner:
LMI offers services also in pre-clinical research in Neurology, Cardiology, and Oncology, as well as radiodiagnostic and radiotherapeutic research and development:
• Radiolabeling setup and optimization for a variety of isotopes
• In vitro and in vivo assay setup and development
• Ligand screening
• Development and optimization of radiopharmaceutical manufacturing processes
• Technology transfers of chemistry and analytical tests
• Design and drafting of clinical study protocols
• Image data analysis
• Reader training and preparation of datasets for transfer to sponsors

Contact
The LMI team is ready to respond to your inquiry regarding our PET imaging radiotracers and clinical trial services. For additional information on LMI’s services as a partner for your clinical trials and/or research, email LMICollaborations[at]life-mi.com
