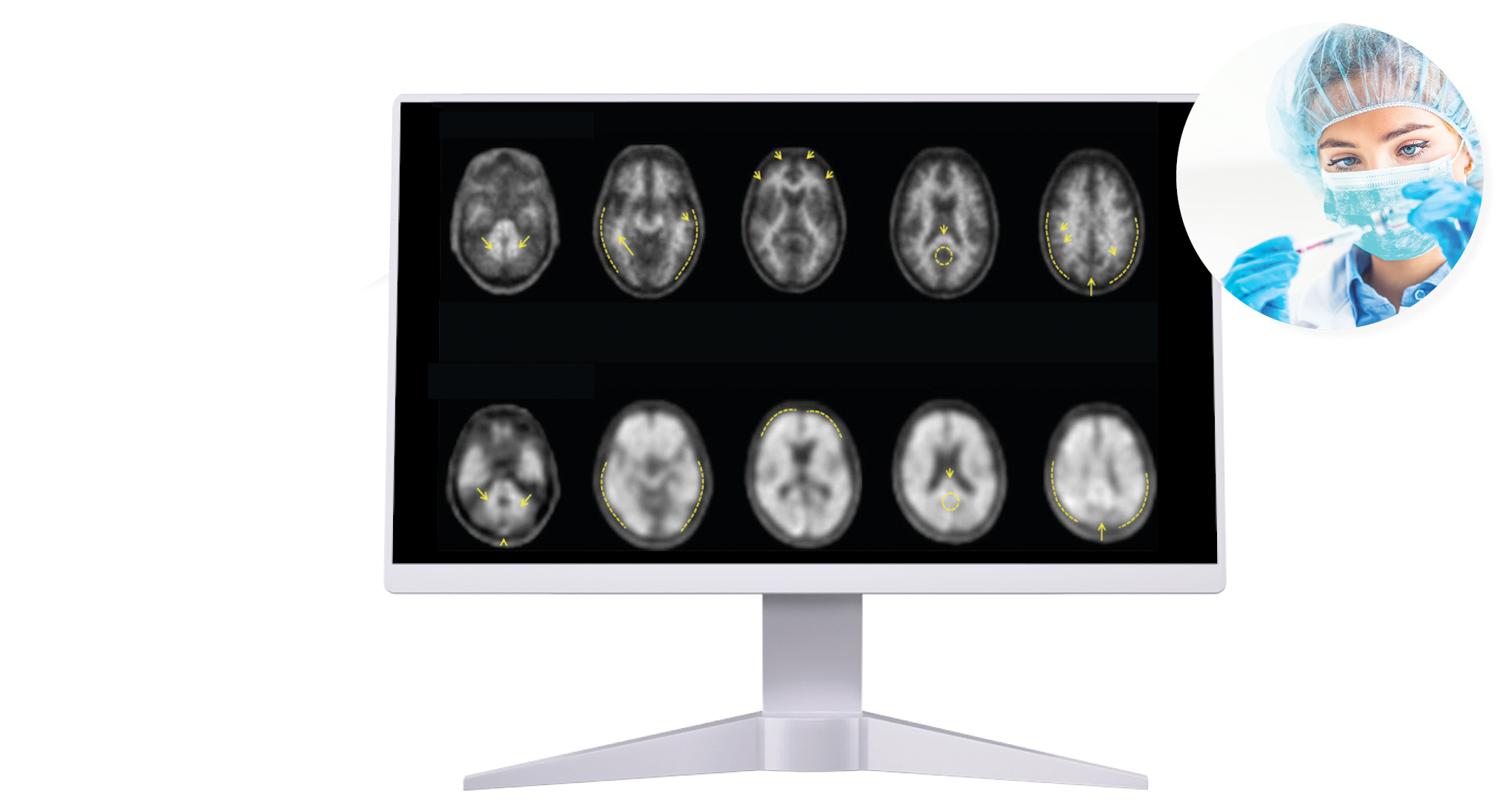
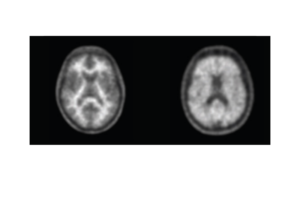
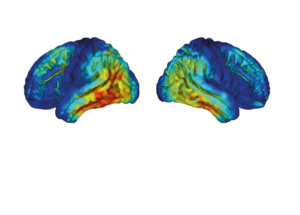
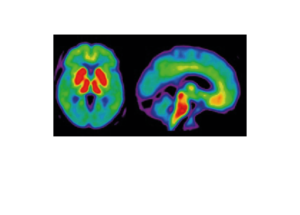
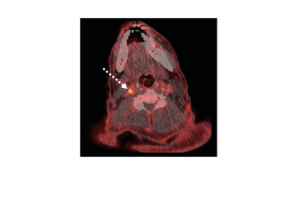
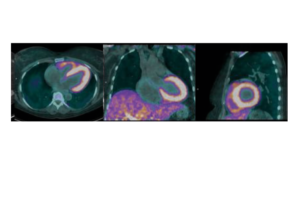
Life Molecular Imaging, a Lantheus Company, is developing a product portfolio of innovative molecular imaging tracers that address major clinical needs in neurology and cardiology.
Life Molecular Imaging, a Lantheus Company
Making the
Making the
Invisible
Visible
Molecular imaging provides unique insights into the human body, enabling physicians to provide an improved diagnosis and personalize patient care