Your Partner for
Molecular Imaging
With the development of novel PET tracers for molecular imaging, Life Molecular Imaging (LMI) is focusing on a key area of modern medicine. LMI strives to be a leader in the field of molecular imaging by developing innovative products that improve early detection and characterization of chronic and life-threatening diseases, leading to better therapeutic outcomes and improved quality of life.

Our Mission
We Provide Innovative PET Tracers
to Reduce the Burden of the Diseases
Our mission is to develop novel PET Tracers in the area of Molecular Imaging that improve early detection and characterization of chronic and life-threatening diseases, leading to better therapeutic outcomes and improved quality of life.
Our History
Life Molecular Imaging (LMI) was founded in 2012 with the acquisition of the molecular imaging research and development portfolio of Bayer Pharma AG by Piramal and is now part of the Life Healthcare Group.
LMI is an international pharma company dedicated to developing and offering novel cutting-edge PET radiopharmaceuticals for imaging of neurodegenerative and cardiovascular diseases. The organization strives to be a leader in the molecular imaging field. Our flagship product Neuraceq® is used to detect the accumulation of amyloid proteins in the brain, which is considered a major cause of Alzheimer’s disease. Neuraceq® is nowadays approved and produced in many countries around the globe.
Our mission is to pioneer innovative PET products that improve early detection and characterization of chronic and life-threatening diseases, leading to better therapeutic outcomes and improved quality of life. By advancing novel PET radiopharmaceuticals for molecular imaging, LMI is focusing on a key field of modern medicine. It’s about making life better for everyone.
Key Milestones
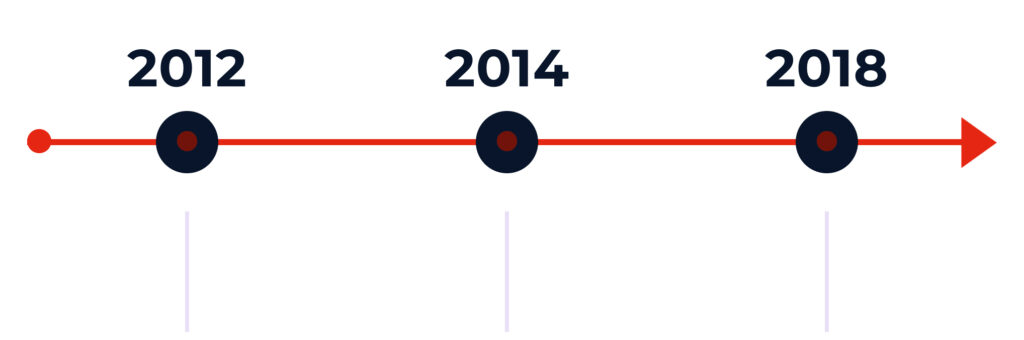
Bayer spins out separate entity – Piramal Enterpises Ltd. becomes new owner
Neuraceq® approved by US & EU regulators
Acquired by Life Healthcare Group
2012
Bayer spins out separate entity - Piramal Enterpises Ltd. becomes new owner
2014
Neuraceq® approved by US & EU regulators.
2018
Aquired by Life's Alliance Medical
Part of the Life Healthcare International Group
An integrated business including research and development laboratories.
Market-leading globally diversified healthcare provider with a large hospital and clinic network in South Africa.
Visit website
LMI develops and commercialises radiopharmaceuticals used in molecular imaging and has a promising pipeline of products in development across the neurogenerative and cardiovascular areas.
Our Partners
We are serving our customers with the help of the most experienced partners in the area of Molecular Imaging.


















Our Leadership Team
The members of Life Molecular Imaging’s leadership team have been working for 20+ years in the pharmaceutical industry.

Ludger Dinkelborg, PhD
Managing DirectorBefore joining Life Molecular Imaging, Dr. Dinkelborg served as Managing Director of Piramal Imaging GmbH, as Director of the Board of Piramal Imaging SA and was the Head of Diagnostic Imaging Research at Bayer HealthCare. He brings more than 30 years of R&D experience in the pharmaceutical industry.
He received his PhD in biology at Heinrich-Heine-University, Düsseldorf, Germany and stayed as guest researcher at the Max-Planck-Institute for Molecular Physiology in Dortmund. He became a fellow of the Konrad-Adenauer-Foundation, attended the Cranfield Schering University course for managers, and the Stanford Executive Program, at Stanford School of Business, Stanford, CA.

Andrew Stephens, MD, PhD
Chief Medical OfficerDr. Stephens has more than 30 years of experience in the pharmaceutical industry, primarily in the areas of neurodegenerative diseases, oncology and diagnostic imaging. Previously, he was Chief Medical Officer at Piramal Imaging and the VP Head Experimental Medicine Oncology/Diagnostic Imaging for Bayer Pharma. He has held positions of increasing responsibility at NeXagen/NeXstar, Gilead, Schering AG, and OSI Pharmaceuticals.
He received his BA cum laude in chemistry from the University of Colorado, Boulder. He also received an MD with honors and a PhD in biochemistry, biophysics and genetics from the University of Colorado, Boulder. Dr. Stephens is Board certified in Internal Medicine and had a clinical practice before entering pharmaceutical development.

Nico Beukman
Chief Commercial Officer Europe, Middle East & AfricaMr. Beukman has more than 25 years of experience in the medical industry with a proven track record for managing strategic growth and execution of sales, marketing and commercial activities. Throughout his career, he has implemented creative and effective solutions for driving revenue growth and improving profitability while managing multinational teams.
Prior to Life Molecular Imaging and Piramal Imaging, Mr. Beukman spent twelve years at Covidien now Curium, serving the company in various leadership roles.
Mr. Beukman earned his National Diploma in Clinical Engineering at Tswane University of Technology in South Africa and more recently completed the Oxford Executive Leadership Program

Colleen Ruby, CNMT, RT(N)
Country Head US and COO Americas & APACMs. Ruby joined the organization in 2014 as Piramal Imaging’s Head of National Sales in the US to identify the market, establish the company’s brand, and to build a salesforce for the launch of Neuraceq® (florbetaben F-18). Ms. Ruby is a Certified and Registered Nuclear Medicine Technologist with more than thirty years of healthcare experience. She holds a degree in Science in Nuclear Medicine Technology and continuously updates her skill set via continuing education courses in health and business applications. Prior to joining the company, Ms. Ruby served as IBA Molecular’s Vice President of Sales and Marketing where she cultivated a professional organization of high-level Sales and Marketing specialists in the Nuclear and Molecular disciplines of the healthcare industry. Ms. Ruby was also previously employed by The University of Pittsburgh Medical Center. She is an active member of the Society of Nuclear Medicine and Molecular Imaging and participates on behalf of the company in the Medical Imaging and Technology Alliance.

Mathias Berndt, PhD
Chief Technology OfficerAt Life Molecular Imaging, Mathias is responsible for our global commercial manufacturing network as well as the technical development of our pipeline assets.
Mathias has more than 15 years year experience in the pharmaceutical industry and radiopharmaceutical development at all stages from discovery to development to New Drug Application and commercialization.
Prior to his current role at Life Molecular Imaging, he was Head of Chemical R&D at Piramal Imaging and held R&D positions at Bayer Healthcare and Schering AG.
Mathias studied chemistry at the Technical University Dresden, received his PhD in organic chemistry from the Free University Berlin and worked as a researcher at the Research Center Rossendorf.

Adrian Karasiewicz
Head of FinanceAdrian Karasiewicz is the Head of Finance at Life Molecular Imaging since May 2021, before served as Financial Controller since he joined the company in 2019. He brings more than 10 years of experience in corporate finance, serving before as an Accountant at Miranda Textiles and Financial Controller at Diehl Group.
He received his Master Degree in Finance and Accounting at Poznań University of Economics, Poland. He continues his development program at Association of Chartered Certified Accountants.

Michel Jongens
Chief Financial Officer - Molecular ImagingMichel Jongens finished his bachelor’s in economics and joined the Dutch Wegener group in 1989 as finance manager. In 1994 he joined InteliCoat Technologies as finance director and he helped build this to be the European head office for this US subsidiary. In 2009 he joined Alliance Medical NE where he restructured the business and took on the role as MD in 2013, before coming CFO of AM NE and LMI in July 2018.

Donna Felker
Chief Legal OfficerMs. Felker has been practicing law in the United States for 18 years, primarily in the life sciences and outsourced services industries. Prior to becoming a lawyer, Ms. Felker spent 14 years in positions of increasing responsibility in domestic (US) and global operations and client management.
Ms. Felker completed her Bachelor Degrees in Business Administration (Financial Management) and Human Resources at the University of Delaware, her Master of Science Degree in Business Administration at Boston University, and her Juris Doctorate at Creighton University School of Law.

Daniela Menzel
Chief People OfficerDaniela Menzel has more than 20 years’ experience in all areas of Human Resources in various positions in different countries and joint the Life Molecular Imaging Group in February 2024. She is an experienced international human resource expert with significant expertise in a broad range of HR topics and worked as strategic partner for the Management for several international pharma companies.
She has a Master of Social Science from the Ruhr University Bochum, a Master of Business Administration from the university of Birmingham and is a Certified Systemic Coach from Humboldt-University, Berlin.
Compliance & Code of Conduct
Life Molecular Imaging is committed to the highest standards of ethical, moral and legal business conduct. Ethical business behaviour is the responsibility of every person in the company and is reflected not only in our relationships with each other but also with our customers, suppliers, clients, contractors, shareholders, and other stakeholders.
Being true to our customers, our environment, and our company, and respecting each other are the fundamental expectations of every Life Molecular Imaging employee and our business partners.
Our culture of compliance, which is fully supported by our senior management, is supported with clear and consistent policies, compliance training, and open communication channels. Life Molecular Imaging has developed its Compliance Program to meet the specific needs of the company and continually assesses the effectiveness of its Compliance Program. We respond quickly to concerns and identify risks, administering corrective action when appropriate.
The Life Healthcare Code of Conduct and related corporate policies are a key component of our commitment to high standards of business and personal ethics.
As member of EFPIA (European Federation of Pharmaceutical Industries Association) Life Molecular Imaging is committed to comply with the EFPIA codes of standard as well as with applicable national laws and regulations for an open and transparent relationship with stakeholders across healthcare including healthcare professionals (HCPs), EU institutions and patient organizations.
Please click for our transparency reporting (as Life Molecular Imaging) for Calendar Year: 2020, 2021, 2022 ,2023.
At Life Molecular Imaging we take violations against our Code of Conduct seriously: we thoroughly investigate known cases and take disciplinary actions where appropriate.
Ethics and Compliance Hotline:
Every Life Molecular Imaging representative (including company personnel and agents) has a duty to report any actual or suspected violations of any laws, regulations, government healthcare program requirements, internal policies and procedures, or inappropriate actions.
Employees, customers, business partners, and third parties may make report concerns either via calling one of the following toll-free call numbers:
- USA: 1 866 317 7033
- UK: 0808 189 1196
- Germany: 08000 181 2227
or by sending an email to: Life@tip-offs.com
All reports submitted to the Integrity Line are handled by a third-party and treated with discretion. LMI does not tolerate any form of retaliation against individuals who report any suspected improper, unethical or illegal conduct.
We at LMI also engage with our various suppliers around the world to ensure they comply with the general principles of LMI’s Supplier Code of Conduct.
Life Molecular Imaging Global Compliance Program
To obtain a copy of the Compliance Program Document, send an email to: compliance@life-mi.com
Annual Declaration of Compliance
California Compliance Law Statement
Life Molecular Imaging “LMI” is committed to compliance laws and regulations that pertains to its business, sales, research, , and marketing practices in the countries in which LMI operates. LMI is in compliance with its Compliance Program (CCP) and Cal. Health & Safety Code §§ 119400-119402. Our Compliance Program contains the elements of an effective compliance program identified in the “Compliance Program Guidance for Pharmaceutical Manufacturers” published by the Office of the Inspector General, U.S. Department of Health and Human Services (“HHS-OIG Guidance”). In addition, LMI has adopted the “Code on Interactions with Healthcare Professionals” published by The Pharmaceutical Research and Manufacturers of America (the “PhRMA Code”) and has policies, procedures and processes designed to help ensure compliance with the PhRMA Code.
In accordance with Chapter 8, Part 15, Division 104 of the California Health and Safety Code (§§ 119400-119402), LMI has established an annual aggregate dollar limit of $2,000 per healthcare professional licensed in California for the following items: 1) meals provided in connection with informational presentations or scientific exchange; 2) educational items intended to enhance patient care (excluding items of minimal monetary value and/or of no independent value (e.g. printedadvertising, reimbursement fact sheets)) and items for the benefit of and distribution to patients/care givers.




