Neuraceq® accurately visualizes β-amyloid neuritic plaques for more personalized patient management.
-
- Scientifically proven ability to detect the presence or absence of β-amyloid neuritic plaques, as demonstrated in a robust clinical trial program
-
- High sensitivity and specificity
-
- Reproducibility and reliability of visual assessment
-
- A demonstrated safety profile with a low radiation dose
-
- A wide, stable, and flexible scanning window
Neuraceq® accurately visualizes β-amyloid neuritic plaques for more personalized patient management.
- Scientifically proven ability to detect the presence or absence of β-amyloid neuritic plaques, as demonstrated in a robust clinical trial program
- High sensitivity and specificity
- Reproducibility and reliability of visual assessment
- A demonstrated safety profile with a low radiation dose
- A wide, stable, and flexible scanning window
Neuraceq® was evaluated in a robust clinical trial program
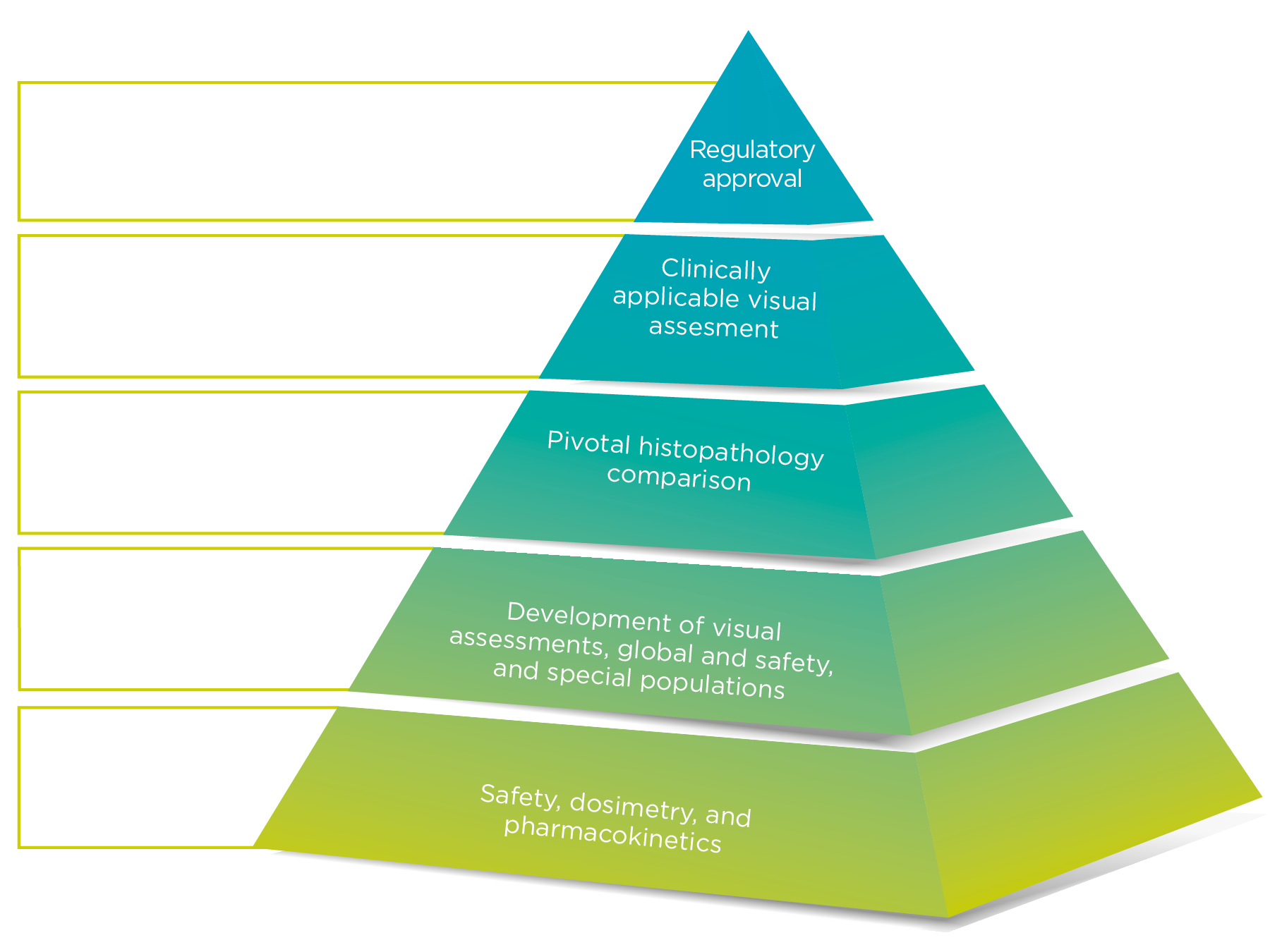
1Neuraceq® [prescribing information]. Life Molecular Imaging; 2021
Highly sensitive β-amyloid detection with both reader training methods (n=82)1
High sensitivity to detect β-amyloid was achieved with both in-person and electronic media training1
Images
Images were taken from 205 end-of-life subjects enrolled and compared with postmortem histopathology as the Standard of Truth (SoT) in 82 subjects
- The histopathologic examination used Bielschowsky silver staining (BSS) of 6 brain regions assessed by a Pathology Consensus Panel masked to all clinical information
Study A
Study A evaluated Neuraceq® PET images with 3 readers who received in-person training
Study B
Study B used 5 readers who underwent training with electronic media
Images
Images were taken from 205 end-of-life subjects enrolled and compared with postmortem histopathology as the Standard of Truth (SoT) in 82 subjects
- The histopathologic examination used Bielschowsky silver staining (BSS) of 6 brain regions assessed by a Pathology Consensus Panel masked to all clinical information
Study A
Study A evaluated Neuraceq® PET images with 3 readers who received in-person training
Study B
Study B used 5 readers who underwent training with electronic media
Study A
a median: 98%; range: 96%-98%. b median: 96%; range: 90%-100%.
Study B
c median: 96%; range: 90%-100%. d median: 77%; range: 47%-80%.
Training for Neuraceq® is provided to ensure proper interpretation of scan images
Study C evaluated the reliability and reproducibility of the clinically applicable image interpretation methodology using the electronic media training; 461 images from previous clinical studies were included from subjects with a range of diagnoses.
Five new readers underwent training with electronic media
Inter-reader agreement across all 5 readers had a kappa coefficient of 0.79 (95% CI 0.77, 0.83)
Intra-reader reproducibility assessed from 46 images (10% of overall sample) ranged from 91% to 98%
Neuraceq® has an established safety profile and a low radiation dose
The safety of Neuraceq® was evaluated in 872 subjects from 9 clinical trials
0 mSv
Neuraceq®
scan
(5.8 mSv)
rec. dose
Head
CT scan
(7.0 mSv)
Whole body
CT scan
(10.0 mSv)
Cardiac
CT scan
(20.0 mSv)
0 MSV
Neuraceq
scan
(5.8 mSv)
rec. dose
Head
CT scan
(7.0 mSv)
Whole
Body scan
(10.0 mSv)
Cardiac
CT scan
(20.0 mSv)
A precise diagnostic tool is needed to reduce the time to accurate diagnosis
- Structural imaging with MRI may not detect early disease or clearly distinguish the etiology of neuronal changes2
- FDG-PET may not be able to detect cortical hypometabolism until several years after detectable β-amyloid pathology has occurred, and may not clarify the reason for the hypometabolism3,4
- Structural and metabolic changes remain surrogate rather than direct markers of dementia pathology4
- Assessment of cognitive decline due to AD depends on detecting the presence or absence of β-amyloid neuritic plaques
- β-amyloid neuritic plaques accumulate early, before the onset of symptoms—but historically could only be visualized via post-mortem histopathology1
- Symptoms alone make the diagnostic interpretation challenging: up to 39% of patients will not have a typical cognitive profile and disparities across cognitive domains occur even between "typical" patients5
FDG-PET, fludeoxyglucose positron emission tomography; MRI, magnetic resonance imaging. 1Fodero-Tavoletti MT, Brockschnieder D, Villemagne VL, et al. In vitro characterization of [18F]-florbetaben, an AB imaging radiotracer. Nucl Med Biol. 2012;39(7):1042-1048. 2Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis. 2011;26(4):627-645 3Prestia A, Caroli A, van der Flier WM, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer’s disease. Neurology. 2013;80(11):1048-1056. 4Mosconi L, Berti V, Glodzik L, Pupi A, DeSanti S, de Leon MJ. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20(3)843-854 5Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43(7):835-845. 6Grossberg GT, Christensen DD, Griffith PA, Kerwin DR, Hung G, Hall EJ. The art of sharing the diagnosis and management of Alzheimer’s disease with patients and caregivers: recommendations of an expert consensus panel. Prim Care Companion J Clin Psyciatry. 2010;12:PCC.09cs00833. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2882814, Accessed January 22, 2014.
Negative and positive scans with Neuraceq® help you clarify the clinical picture and plan the path ahead
Neuraceq® delivers valuable information for greater confidence in diagnosis
and more personalized patient management.
- Negative Neuraceq® PET scans allow you to consider alternative causes of cognitive impairment
- A positive Neuraceq® PET scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition3
- Neuraceq® is an adjunct to other diagnostic evaluations and enables physicians and patients to plan accordingly
- The safety and efficacy of Neuraceq® has not been fully established for the prediction of the development of dementia or other neurologic conditions
- A positive scan alone does not establish the diagnosis of AD or any other cognitive disorder, particularly in the absence of cognitive impairment
- Symptoms alone make the diagnostic interpretation challenging: up to 39% of patients will not have a typical cognitive profile and disparities across cognitive domains occur even between "typical" patients5
3Neuraceq® [prescribing information]. Life Molecular Imaging; 2021 3Prestia A, Caroli A, van der Flier WM, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer’s disease. Neurology. 2013;80(11):1048-1056.
Neuraceq® delivers sensitive and reliable detection of β-amyloid neuritic plaques
Neuraceq® is an accurate diagnostic tool to aid in the evaluation of cognitive decline1
• Neuraceq® reveals β-amyloid neuritic plaques by uptake in different brain regions, thereby allowing an estimate of plaque density by degree of uptake1
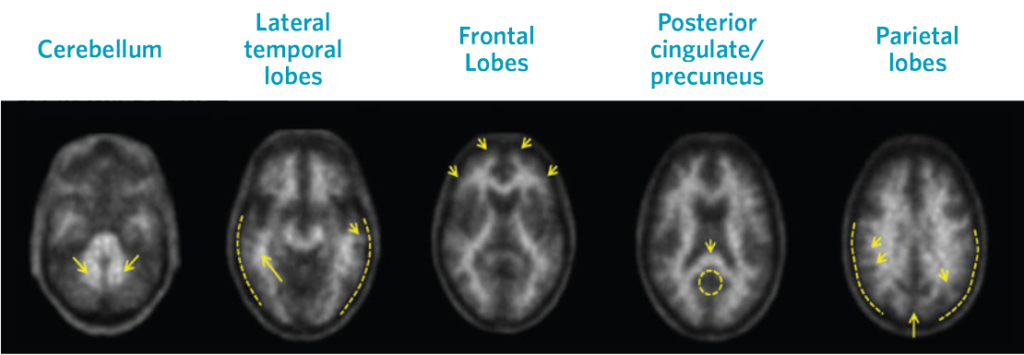
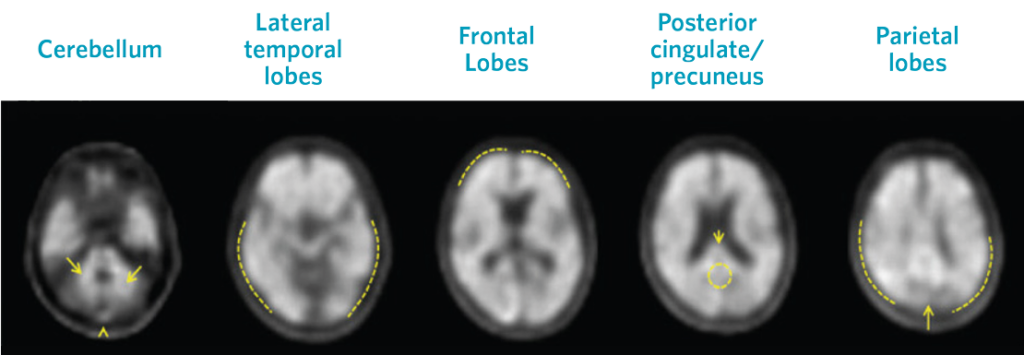
Visual assessment is based on the systematic comparison of tracer uptake in gray and white matter, focusing on specific cortical regions, as seen in black and white scan images.
Negative Scans
Top row images are negative scans, corresponding to lower tracer uptake in gray matter than white matter, and reduced likelihood that cognitive decline is a result of AD
Positive Scans
Images show positive scans with equal or higher tracer uptake in gray matter than white matter and moderate to frequent β-amyloid neuritic plaques
1Neuraceq® [prescribing information]. Life Molecular Imaging; 2021
Neuraceq® scan reports can help facilitate dialogue between physicians, patients, and caregivers
While the actual diagnosis is made by reading the black and white scans, useful reports provided to you by your patient’s imaging specialist include an accompanying written assessment.
The Neuraceq® scan is convenient for patients
Injection
Neuraceq® injection

45+ Minutes
Wait at least 45 minutes before PET scan can begin
During the scan
The PET scan lasts 15 to 20 minutes

After the scan
The PET scan should be completed within about 2 and a half hours after injection
Frequently Asked Questions about Neuraceq®
Neuraceq® was FDA approved in March 2014 and is available in many imaging centers across the US.
Contact Us for specific details on Neuraceq® availability in your area or send an email to sales@life-mi.com.
Neuraceq® can give you important information in cases where Alzheimer's disease is suspected. If the scan is positive, it means that beta amyloid plaques are present in the brain. If the scan is negative, it means that beta amyloid plaques are not present, and another diagnosis can be considered.
Early detection and intervention can benefit patients. Neuraceq® alone does not diagnose AD, it should be used in conjunction with other tests. Having a diagnosis gives patients the opportunity to plan ahead. Pharmacological and non-pharmacological interventions can begin sooner. Knowing a diagnosis gives patients the opportunity to take part in clinical trials.
Our patient and caregiver information can be viewed on our patient and caregiver page. If you would like a printed copy of the brochure please contact 617-725-0070 or send an email to info@life-mi.com.
Contact Us for more information.
Important Safety Information
Risk for Image Misinterpretation and Other Errors
Neuraceq® can be used to estimate the density of β-amyloid neuritic plaque deposition in the brain. Neuraceq® is an adjunct to other diagnostic evaluations. Neuraceq® images should be interpreted independent of a patient’s clinical information. Physicians should receive training prior to interpretation of Neuraceq® images. Following training, image reading errors (especially false positive) may still occur. Additional interpretation errors may occur due to, but not limited to, motion artifacts or extensive brain atrophy.
Radiation Risk
Administration of Neuraceq®, similar to other radiopharmaceuticals, contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. It is important to ensure safe handling to protect patients and health care workers from unintentional radiation exposure.
Most Common Adverse Reactions
In clinical trials, the most frequently observed adverse drug reactions in 872 subjects with 1090 Neuraceq® administrations were injection/application site erythema (1.7%), injection site irritation (1.1%), and injection site pain (3.4%).